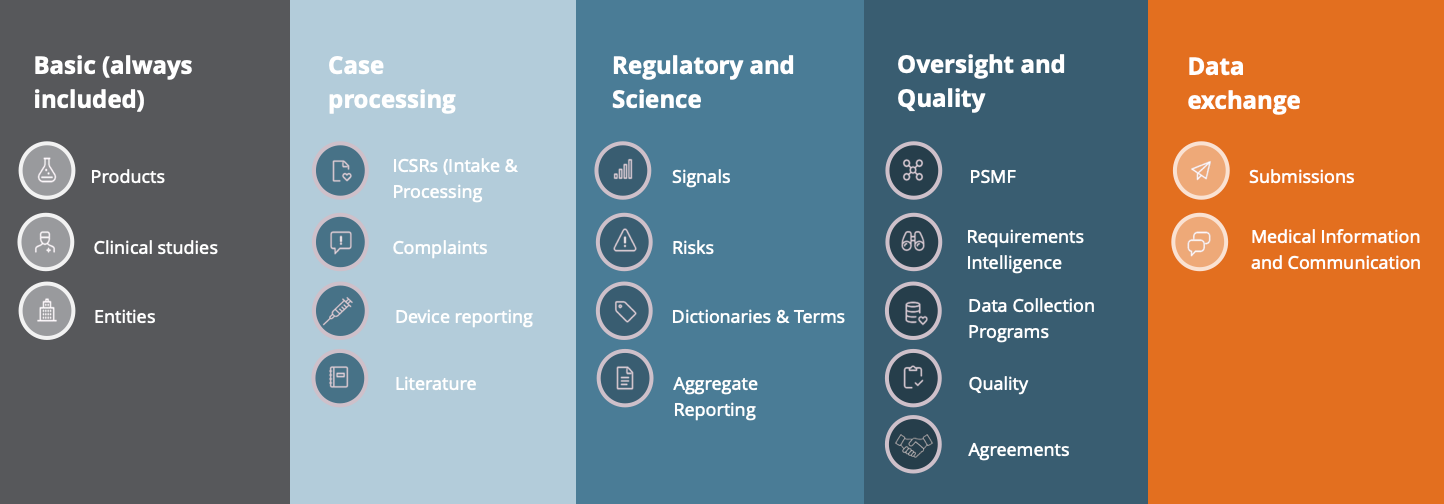
Safety Database
Centralizing all safety data in a single system, managed and executed by LINK Medical, facilitates smooth reporting and ensures seamless compliance with pharmacovigilance regulations. This integrated approach optimizes data integrity and accessibility and enhances the efficiency of safety monitoring and regulatory submissions.
Together with Insife we collaborate on the development on our safety database, HALOPV. The partnership is a cooperation where Insife develop and build the technical infrastructure, and LINK Medical deliver the expert knowledge of safety and the workflow of SUSARs.