Study Start-Up Group
Comprehensive support for all types of studies
The Study Start-Up (SSU) Group is involved in all clinical study applications submitted to Competent Authorities (CAs) and/or Ethics Committees (ECs) for our full-service studies and can also support clients that only need help with submissions and interactions with CAs or ECs.
The SSU group is managed by a Group Manager SSU and handles clinical study and clinical investigation/performance study submissions for Investigational Medicinal Products (IMPs) and Medical Devices (MDs) including in vitro diagnostic (IVD).

Our Geographical Area
Within our geographical area we work with all types of studies (interventional, observational, and other studies e.g. retrospective data/sample collection) and engage staff from Clinical Operations and Regulatory departments as Local Regulatory contact Regulatory Lead.
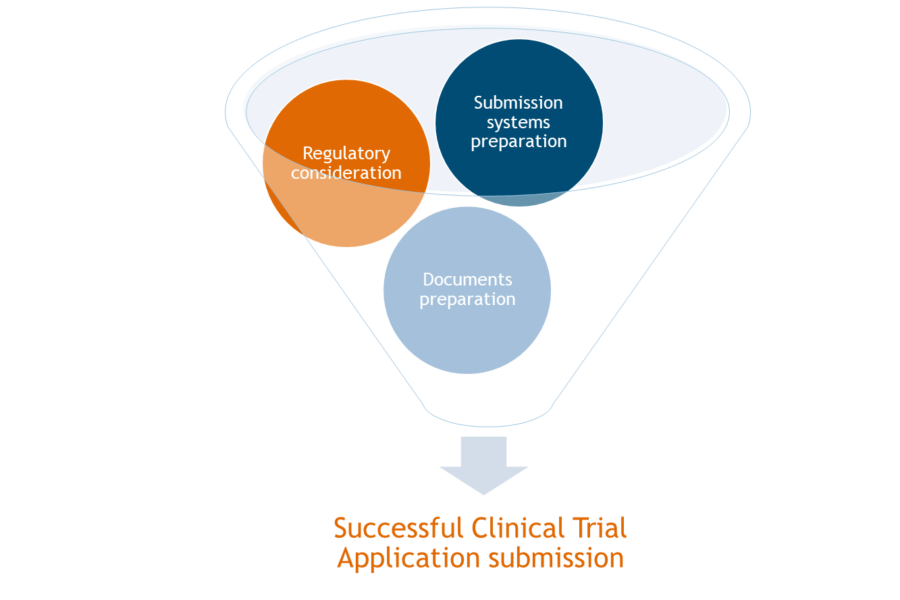
Regulatory Considerations:
System preparation/Maintenance:
Document preparation: