Therapeutic Areas and Experience
Clinical trials delivered with integrity
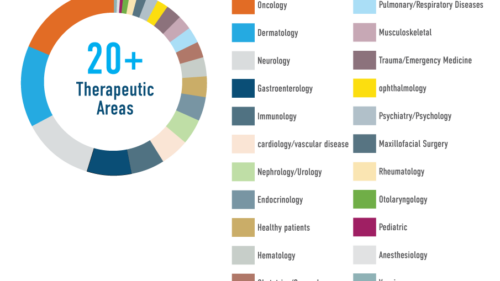
LINK Medical has extensive experience of diverse therapeutic areas, developed and fine-tuned over the course of delivering more than 590 clinical trials.
The performance of these trials, across all phases, has allowed us to continuously widen our proficiency in a variety of indications and complexities, maximizing our ability to tailor our services to your specific requirements. Our success can be attributed to the company´s highly qualified senior leadership and the dedication and expertise of our delivery teams.
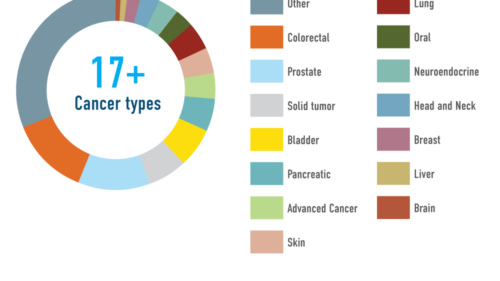
Oncology experts
Vast experience in Oncology trials, including phase I
It’s important to find a partner CRO that has specific experience in the indication and the phase of the studies needed, so that you are well prepared for the particular challenges that can arise and can handle them effectively.
LINK Medical knows exactly why oncology trials are complex. So we have active collaborations with clinics for early trials in oncology, experts in oncology trial design and a huge biostatistical team to make sure that the design and power can be achieved.
We continuously build on our strong background in conducting oncology trials, involving oncology experts already from the design and feasibility stage to make sure the trial is performed in an optimal way. Being aware that state-of-the art of early trial design and early development strategy are evolving we make a point of including up-to-date plans in our proposals for oncology early development programs.

Our Technologies
Expertise and Technology are one of LINK Medicals’ strengths
The technologies we use are rigorously selected and maintained so that we can support studies or registries running all over the world and a large variety of trials.
As Master Users of Viedoc, we possess a distinct edge which means, amongst other things, that we can be particularly time- and cost-effective in helping you develop a study design to achieve your critical endpoints.
