 Hedda Magnusson, Director of Clinical Operations, Rikard Reneland, Medical Director, and Mario Baban, Group Manager, Clinical Data Management
Hedda Magnusson, Director of Clinical Operations, Rikard Reneland, Medical Director, and Mario Baban, Group Manager, Clinical Data Management
The interest in developing therapies for diseases affecting the central nervous system, (CNS) has peaked recently. After the positive results from clinical studies testing two experimental drugs to treat Alzheimer’s disease, a lot of other research groups and companies have started to intensify their work within the field.
The great news for patients and their families is that the ongoing work in developing treatments for CNS-diseases is of high priority on a global scale. It is an area of high activity with many world-class research institutions on board.
The challenges of developing CNS-therapies
However, the risk of failure within new therapies for CNS-diseases is very high compared to many other therapeutic areas. There are several reasons for this, and it is important that anyone in the process of investing a great deal of time and resources into developing novel CNS-therapies are aware of these challenges and even more importantly, have strategies in place to overcome them.
The most common challenges are based on the difficulty to generate findings in the area of CNS-diseases, that can be translated into a successful treatment. This is due in part to the difficulty in identifying the appropriate measurement and interpreting the results when working with patients with dementia and other CNS conditions. Often, the caregivers are important by contributing complementary data.
Another difficulty is that trials in this indication tend to be long, as the clinical results can only be observed after long follow-up, and because of a lack of convenient and predictive biomarkers.
Overcoming challenges
What can be done to avoid the most common challenges? The strongest recommendation and way forward is most certainly to partner with a team of experts who can guide the project through every milestone.
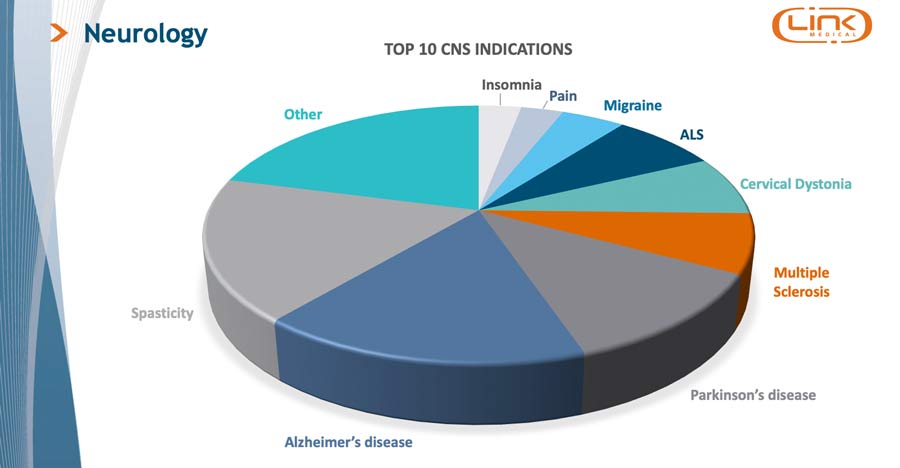
LINK Medical is a Nordic CRO with experience and know-how in the field of developing novel CNS-therapies. With experience on the top CNS indications like Alzheimer’s, Parkinson’s, MS, Insomnia, and more, LINK understands the risks and challenges and provides the quality assurance, clinical data management, and regulatory guidelines necessary by thinking ahead of the challenges at hand.
“Our advantage is that we are a one source partner. We have all the expertise in our own organisation to prepare for and perform the clinical trials in all stages. We have a solid understanding of how these types of studies must be performed to reach success, we know the common pitfalls from experience, and we can help our clients avoid them. Therefore, we can also say that we can help our clients speed up the process”, says Rikard Reneland, Medical Director at LINK Medical.
The advantages of smart systems and experienced partner
These types of studies generate large amounts of data. How is it possible to make sense of it all?
“We have many years of clinical data management experience specific to CNS studies. The knowledge and experience gained comes from working with various phases in large multi-national studies. LINK has technical eClinical solutions to solve the challenges our customers are facing. Regardless of if data is submitted directly by a patient, clinic or by site-patient interaction with the help of secure video calls. The seamless integration between the addons in the system, allows our customers to capture, view and analysed the data in real time. LINK’s expertise in addition to using best-in-class and proven modern technologies, can contribute to successfully performing and efficiently conducting studies”, says Mario Baban, Group Manager Clinical Data Management, LINK Medical.
Key considerations for efficient outcomes
CNS studies require not only helping the patient but also accommodating the caregiver, therefore using every new technology available is critical. With every household having electronic devices available today and the smart use of online tools, many tasks in a clinical study can be done remotely. Following are a few key considerations:
- Patient Recruitment – recruitment is always a highly demanding process, and it takes a lot of resources to achieve the number of patients required. Having a network of KOLs and known sites is highly important when starting up a clinical trial. Targeted advertising through different media can also be an important tool for patient recruitment. By offering a decentralised approach, you increase the possibilities for more patients to take part in a study.
- Screening tools for efficient patient enrollment: the use of web-based pre-screening and online questionnaires, when an advertisement is used for enrollment, helps filter through the number of patients coming to the clinic. This greatly supports the sites by reducing the time spent in the pre-screening and recruitment process.
- Patient retention: While some visits must be performed on-site, having the option for remote follow-ups helps stay compliant with the required visits during the study. Using a system that provides automated reminders on activities and visits also increases the adherence and patient retention.
- Cleaner data: Capturing complete and high-quality data for CNS studies historically has been a huge challenge. Having the right digital tools that ease and harmonize how the data is entered is key. Creating tailored forms and scales can help in having a consistent way to enter data and in turn improve the way the data is measured and interpreted.
- A flexible mindset: Each study is unique and a CRO with extensive experience, the latest technologies and an adaptable process is essential. LINK Medical has a long and close collaboration with Viedoc™, a leading Swedish provider of digital and decentralized software solutions for clinical studies. Having hands-on experience with different types of studies and as an active user of eClinial software, LINK collaborates with Viedoc by taking an active role in the development of new solutions based on industry needs and supports Viedoc by testing and perfecting the new solutions.
Using the options that support efficiency can significantly reduce time and budget while supporting the patients and ultimately accelerating the new treatments into the market.


